Chemistry Solved MCQs for all competitive exam
रसायन विज्ञान से सम्बंधित बहुविकल्पीय प्रश्न उत्तर कुंजी सहित
Welcome to our exclusive collections of chemistry multiple choice questions(MCQs) with answers. All questions are of multiple choice type. In these multiple choice questions MCQs sets we are going to share most important questions related to chemistry. These questions are very helpful for those candidates and aspirants who are preparing for the various government and non government exam as well as in interview. e.g. CTET, TET, HPAS, UPSc, State PSC, Army, Patwari, Samvida, Teacher recruitment test, UPSC, SSC, CGL, Police, SI, CDS, MAT, SSC 10+2, CLAT, NIFT, SBI, IBPS PO, SBIPO, PO, RRB, IBPS Clerk, Vyapam, CDSE, SBI Clerk etc.
रसायन विज्ञान से सम्बंधित बहुविकल्पीय प्रश्नो के इस सेट में कई महत्वपूर्ण प्रश्न उत्तर कुंजी सहित दिए गए है जो कई प्रतियोगी परीक्षाओ में पूछे गए प्रश्न का समावेश किये हुए है. लगभग सभी प्रतियोगी परीक्षाओ में रसायन विज्ञान से सम्बंधित बहुविकल्पीय प्रश्न पूछे जाते है हम रसायन विज्ञान से सम्बंधित महत्वपूर्ण बहुविकल्पीय प्रश्न हिंदी में उत्तर कुंजी सहित दे रहे हे जो उन सभी स्टूडेंट्स के लिए महत्वपूर्ण है जो रसायन विज्ञान से सम्बंधित बहुविकल्पीय प्रश्नो की जानकारी लेना चाहते है तथा कई प्रतियोगी परीक्षाओ की तैयारी कर रहै है | ये महत्वपूर्ण बहुविकल्पीय उत्तर कुंजी सहित प्रश्न उन सभी लोगो के लिए वरदान साबित होंगे ।
Chemistry MCQs for all Competitive Exams|रसायन विज्ञान से सम्बंधित बहुविकल्पीय प्रश्न उत्तर कुंजी सहित
Chemistry Solved MCQs| रसायन विज्ञान से सम्बंधित महत्वपूर्ण बहुविकल्पीय प्रश्न उत्तर कुंजी सहित-SET-45
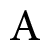 Greater than one and greater than one
Greater than one and greater than one
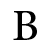 Less than one and greater than one
Less than one and greater than one
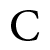 Less than one and less than one
Less than one and less than one
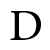 Greater than one and less than one
Greater than one and less than one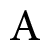 Penta ammine nitro cobalt (III) ion
Penta ammine nitro cobalt (III) ion
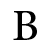 Penta ammine nitro cobalt (IV) ion
Penta ammine nitro cobalt (IV) ion
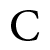 Penta ammine nitrito cobalt (IV) ion
Penta ammine nitrito cobalt (IV) ion
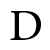 Penta ammine nitrito cobalt (III) ion
Penta ammine nitrito cobalt (III) ion
You May Also Like These Posts
HP-TET-Arts Old Solved Papers
- HP-TET-ARTS-2012 SOLVED PAPER
- HP-TET-ARTS-2013 SOLVED PAPER
- HP-TET-ARTS-2014 SOLVED PAPER
- HP-TET-ARTS-2015 SOLVED PAPER
- HP-TET-ARTS-2016 SOLVED PAPER
- HP-TET-ARTS-2017 SOLVED PAPER
- HP-TET-ARTS-2018 SOLVED PAPER
- HP-TET-ARTS-JUNE-2019 SOLVED PAPER
- HP-TET-ARTS-NOV-2019 SOLVED PAPER
- HP-TET-ARTS-JUNE-2020 SOLVED PAPER
- HP-TET-ARTS-NOV-2020 SOLVED PAPER
- HP-TET-ARTS-JUNE-2021 SOLVED PAPER
- HP-TET-ARTS-NOV-2021 SOLVED PAPER
- HP-TET-ARTS-JUNE-2022 SOLVED PAPER
- HP-TET-ARTS-NOV-2022 SOLVED PAPER
- HP-TET-ARTS-JUNE-2023 SOLVED PAPER
HP-TET-NON-MEDICAL SOLVED PAPERS
- HP-TET-NM-2012 SOLVED PAPER
- HP-TET-NM-2013 SOLVED PAPER
- HP-TET-NM-2014 SOLVED PAPER
- HP-TET-NM-2015 SOLVED PAPER
- HP-TET-NM-2017 SOLVED PAPER
- HP-TET-NM-2018 SOLVED PAPER
- HP-TET-NM-JUNE-2019 SOLVED PAPER
- HP-TET-NM-NOV-2019 SOLVED PAPER
- HP-TET-NM-JUNE-2020 SOLVED PAPER
- HP-TET-NM-NOV-2020 SOLVED PAPER
- HP-TET-NM-JUNE-2021 SOLVED PAPER
- HP-TET-NM-NOV-2021 SOLVED PAPER
- HP-TET-NM-JUNE-2022 SOLVED PAPER
- HP-TET-NM-NOV-2022 SOLVED PAPER
- HP-TET-NM-JUNE-2023 SOLVED PAPER
HP-TET-MEDICAL SOLVED PAPERS
- HP-TET-MEDICAL-2012 SOLVED PAPER
- HP-TET-MEDICAL-2013 SOLVED PAPER
- HP-TET-MEDICAL-2014 SOLVED PAPER
- HP-TET-MEDICAL-2015 SOLVED PAPER
- HP-TET-MEDICAL-2016 SOLVED PAPER
- HP-TET-MEDICAL-2017 SOLVED PAPER
- HP-TET-MEDICAL-2018 SOLVED PAPER
- HP-TET-MEDICAL-JUNE-2019 SOLVED PAPER
- HP-TET-MEDICAL-NOV-2019 SOLVED PAPER
- HP-TET-MEDICAL-JUNE-2020 SOLVED PAPER
- HP-TET-MEDICAL-NOV-2020 SOLVED PAPER
- HP-TET-MEDICAL-JUNE-2021 SOLVED PAPER
- HP-TET-MEDICAL-NOV-2021 SOLVED PAPER
- HP-TET-MEDICAL-JUNE-2022 SOLVED PAPER
- HP-TET-MEDICAL-NOV-2022 SOLVED PAPER
- HP-TET-MEDICAL-JUNE-2023 SOLVED PAPER
HP-JBT ENTRANCE SOLVED PAPERS
- HP-JBT ENTRANCE SOLVED PAPERS
- HP-JBT ENTRANCE EXAM-2010 SOLVED PAPER
- HP-JBT ENTRANCE EXAM-2014 SOLVED PAPER
- HP-JBT ENTRANCE EXAM-2015 SOLVED PAPER
- HP-JBT ENTRANCE EXAM-2017 SOLVED PAPER
- HP-JBT ENTRANCE EXAM-2018 SOLVED PAPER
- HP-JBT ENTRANCE EXAM-2019 SOLVED PAPER
- HP-JBT ENTRANCE EXAM-2020 SOLVED PAPER
- HP-JBT ENTRANCE EXAM-2021 SOLVED PAPER
- HP-JBT ENTRANCE EXAM-2022 SOLVED PAPER
- HP-JBT ENTRANCE EXAM-2023 SOLVED PAPER
HP-TET-JBT/D.El.Ed SOLVED PAPERS
- HP-TET-JBT SOLVED PAPERS
- HP-TET-JBT-2013 SOLVED PAPER
- HP-TET-JBT-2015 SOLVED PAPER
- HP-TET-JBT-2017 SOLVED PAPER
- HP-TET-JBT-2018 SOLVED PAPER
- HP-TET-JBT-JUNE-2019 SOLVED PAPER
- HP-TET-JBT-NOV-2019 SOLVED PAPER
- HP-TET-JBT-JUNE-2020 SOLVED PAPER
- HP-TET-JBT-NOV-2020 SOLVED PAPER
- HP-TET-JBT-JUNE-2021 SOLVED PAPER
- HP-TET-JBT-NOV-2021 SOLVED PAPER
HP-TET-LT SOLVED PAPERS
- HP-TET-LT SOLVED PAPERS
- HP-TET-LT-2014 SOLVED PAPER
- HP-TET-LT-2015 SOLVED PAPER
- HP-TET-LT-2016 SOLVED PAPER
- HP-TET-LT-2017 SOLVED PAPER
- HP-TET-LT-2018 SOLVED PAPER
- HP-TET-LT-JUNE-2019 SOLVED PAPER
- HP-TET-LT-NOV-2019 SOLVED PAPER
- HP-TET-LT-JUNE-2020 SOLVED PAPER
- HP-TET-LT-NOV-2020 SOLVED PAPER
- HP-TET-LT-JUNE-2021 SOLVED PAPER
- HP-TET-LT-NOV-2021 SOLVED PAPER
HP-TET-SHASTRI SOLVED PAPERS
- HP-TET-SHASTRI SOLVED PAPERS
- HP-TET-SHASTRI-2014 SOLVED PAPER
- HP-TET-SHASTRI-2015 SOLVED PAPER
- HP-TET-SHASTRI-2016 SOLVED PAPER
- HP-TET-SHASTRI-2017 SOLVED PAPER
- HP-TET-SHASTRI-2018 SOLVED PAPER
- HP-TET-SHASTRI-JUNE-2019 SOLVED PAPER
- HP-TET-SHASTRI-NOV-2019 SOLVED PAPER
- HP-TET-SHASTRI-JUNE-2020 SOLVED PAPER
- HP-TET-SHASTRI-NOV-2020 SOLVED PAPER
- HP-TET-SHASTRI-JUNE-2021 SOLVED PAPER
- HP-TET-SHASTRI-NOV-2021 SOLVED PAPER

